

We need our cell phones to last as long as possible while we are texting people while out to dinner with our significant others. Most consumer electronics with rechargeable batteries use Cobalt Oxide because it has a high specific energy density.

Think Merlin, but with better access to exotic materials. Presto! Now we have a battery that is more stable with high specific energy (capacity).
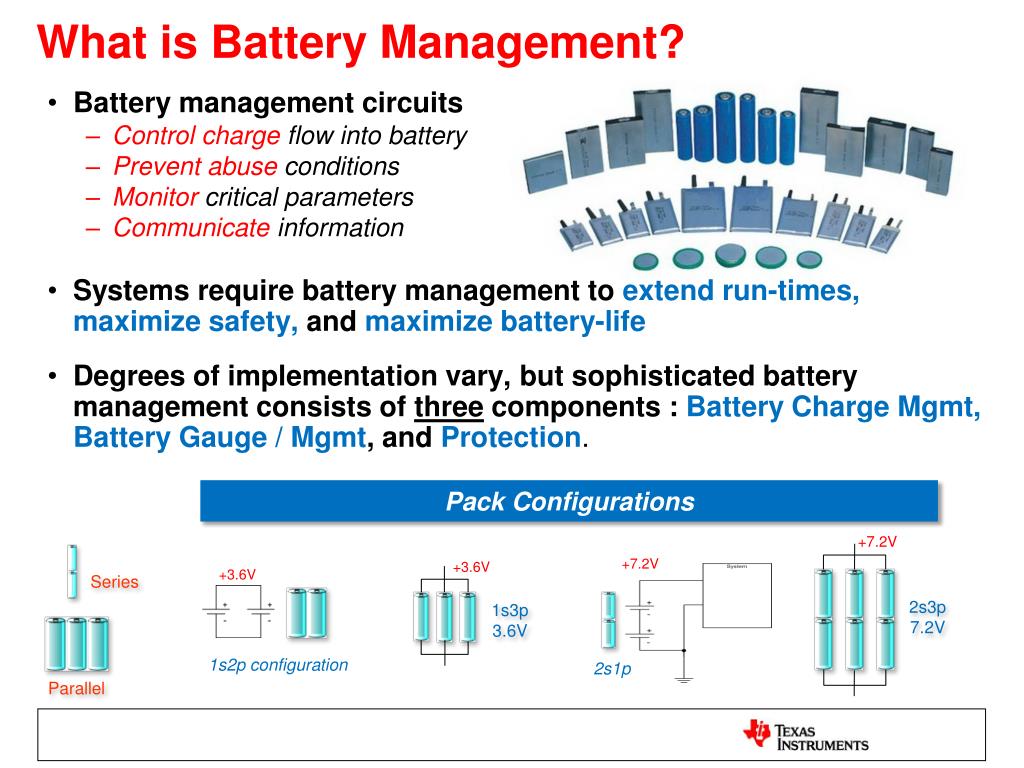
So, engineers mix in a little manganese because it has low internal resistance. Nickel, if used by itself, has a high energy rating but is unstable. Nickel Manganese Cobalt Oxide (NMC) batteries use nickel as the primary metal on the battery cathode. The most common ones used in residential storage applications - Nickel Manganese Cobalt Oxide (NMC) and Lithium Iron Phosphate (LFP) are explained below. There are five or six main types of Li-ion batteries. To the laymen, it is an incoherent jumble of the periodic table, but the engineers were quite clever in combining these materials together to take advantage of the strengths of each element. You never get something for nothing.Ī quick Internet search reveals Li-ion battery types by their chemical designation: LiNixMn圜ozO2, LiFePO4, and LiNiCoAlO2 to name a few. The goal is to cram as many peanuts into the smallest bag possible however, this process does have limitations. When digging into the various kinds of Li-ion batteries, it seems that battery chemists are having fun playing around with different kinds of metals to see how each one reacts. There are many types of lithium-ion batteries, each engineered with their own different materials. It is a myth, even lead acid doesn't suffer from it. By the way, this memory effect is only applicable to the nickel-cadmium batteries from the '70s and 80s but somehow applied to every other battery on the market, even to this day. Li-ion batteries do not suffer (much) from “memory” losses which means they can sit fat, dumb and happy at any point in their charge cycle and recover to a 100% state of charge. They have a higher energy density than traditional batteries, which means you can pack more power into a smaller footprint, like cramming 10 pounds of peanuts into a 5-pound bag. Lithium-ion (Li-ion, or LI) batteries have been around since the '70s and are used in many consumer electronics, e-bikes and e-scooters, and electric cars and trucks. Although there are many promising battery chemistries and technologies, as well as the tried-and-true lead-acid variants, this article focuses on lithium since it is the dominant player in global residential, commercial, and utility-scale storage. As solar and storage gain more momentum in the public eye, more questions about the current and future battery technologies are inevitable.


 0 kommentar(er)
0 kommentar(er)
